Introduction
Sulfuric acid is a highly versatile compound extensively used in various industries. It serves as a fundamental building block for thousands of other chemical compounds produced in chemical plants. A significant portion of sulfuric acid is consumed in the production of sulfate fertilizers, such as ammonium sulfate. Additionally, it plays a crucial role in the manufacturing of phosphoric acid, another essential component in phosphate fertilizers. Furthermore, sulfuric acid finds extensive applications in the metallurgical industry for metal processing.
Production Processes
The main method of producing sulfuric acid is the contact process. In this process, sulfur is first converted to sulfur dioxide (SO₂). The sulfur dioxide is then oxidized to sulfur trioxide (SO₃) in the presence of a vanadium pentoxide (V₂O₅) catalyst. Finally, the sulfur trioxide combines with water to produce sulfuric acid.
Production of Sulfuric Acid by Direct Contact Process
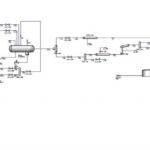
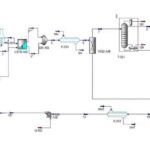
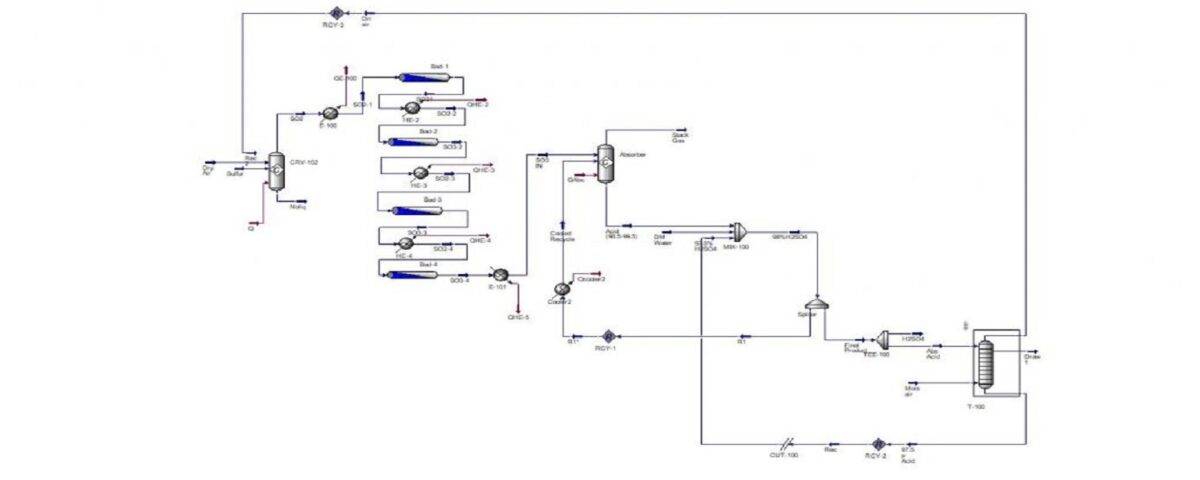
The contact process is a primary industrial method for producing sulfuric acid. In this process, high-purity sulfur (typically 99%) is employed as the feedstock. The sulfur is melted in concrete tanks using steam coils at a temperature of 125°C. Subsequently, it is pumped into a combustion furnace where it burns to form sulfur dioxide (SO2). The resulting SO2 gas is then directed to a heat exchanger and subsequently reacts with vanadium pentoxide, a catalyst, to form sulfur trioxide (SO3).
Within absorption towers, the sulfur trioxide (SO3) is absorbed by 98% sulfuric acid, resulting in the formation of oleum. This oleum is immediately transferred to a circulation tank. The term “circulation tank” is used because the sulfuric acid within this tank, as well as in the absorption towers, is continuously circulated, forming a closed production cycle.
By adding water to the tank, the oleum is diluted, producing sulfuric acid with a concentration exceeding 98%. The resulting acid is then transferred to storage tanks via specialized pipelines. This process yields sulfuric acid with a concentration greater than 98%.
S + O2 → SO2 + q
SO2 +1/2 O2 → SO3 + q
SO3 +H2O →H2SO4 + q
H2SO4 + SO3→ H2S2O7
Currently, there are more than 50 companies producing sulfuric acid in IRAN, all of which use the contact method for their production.

Application of Sulfuric Acid
As mentioned in the first part of this article, the primary use of sulfuric acid across various industries is in agriculture, particularly for the production of phosphate fertilizers and to alleviate soil salinity. Approximately 60% of sulfuric acid consumption is in fertilizer manufacturing plants, where it is used to produce aluminum sulfate in conjunction with ammonia. Additionally, by adding sulfuric acid to phosphate rock, NSP (normal superphosphate) fertilizer is produced, and phosphoric acid is generated through the wet process with the aid of sulfuric acid. A small percentage of this acid is used directly in the fertilizer industry as a liquid fertilizer.
Application of Sulfuric Acid in Leather Tanning
Sulfuric acid or sulfonic acid, with its strong hydrolysis power, acts as a solvent for fats and lipids. Due to this property and its ability to cleanse and remove contaminants, it is widely used. In the leather tanning industry, it is employed to remove hair and grease from hides using sodium hydrosulfide present in the acid. After this stage, the leather becomes brittle, posing challenges in dyeing leather products. To address this issue and prepare the leather for coating and dyeing, sulfuric acid, sodium bisulfite, and other substances are used in the leather industry.
Application of Sulfuric Acid in the Steel Industry
In the steel industry, sulfuric acid is used to produce a byproduct, ammonium sulfate, by recovering ammonia-containing gases. It is also employed in metal oxide operations for degreasing through a process known as “picking.”
.In this project, the production process of sulfuric acid is simulated by proximity method with the help of Aspen Hysys software