Introduction
Over the past three decades, the management of Petrosakht-Chehelsoton Company has played a significant role in the petrochemical downstream industry by establishing and launching various units. With the expansion of their activities in 2009, the managers decided to establish an engineering and technical company, Petrosakht-Chehelsoton, to execute large projects in the oil, gas, refining, petrochemical, power generation, steel, and mining sectors. Our mission is to achieve EPC projects at competitive prices, foster a reliable and trustworthy collaboration environment, and complete projects within a reasonable timeframe while ensuring satisfactory and acceptable quality.
Supported by our experienced management, technical, engineering, and commercial teams, along with the existing workshop facilities at the factory, Petrosakht-Chehelsoton is distinguished by its seamless integration of engineering, procurement, and construction in a cohesive manner, without dependence on other independent companies. This integrated approach allows for significant cost reduction and time-saving in project execution.
Aromatics
Aromatics, or aromatic compounds, are a group of chemical compounds characterized by their stability and unique chemical properties due to the conjugated pi electron system in a cyclic structure. Aromatic compounds exhibit distinct stability known as aromaticity, created from a system of alternating double and single bonds forming a ring.
The primary sources for producing aromatics include catalytic reforming processes and pyrolysis gasoline produced in cracking units with steam. Catalytic reforming is commonly used in oil refineries to produce high-octane gasoline. Steam cracking is used to produce light olefins such as ethylene, and when using liquid feedstock in these units, a byproduct rich in aromatics known as pyrolysis gasoline (Pyrolysis Gasoline) is produced, from which benzene, toluene, and xylenes can be extracted. Typically, aromatic compounds are initially separated from non-aromatics using extractive distillation, followed by distillation further separating benzene, toluene, and xylenes.
Common Aromatic Compounds
– Benzene (C6H6): The simplest aromatic hydrocarbon, used as a feedstock for many chemicals.
– Toluene (C7H8): A methyl-substituted benzene, used as an industrial solvent and precursor for other chemicals.
– Naphthalene (C10H8): Composed of two fused benzene rings, commonly used in mothballs.
– Phenol (C6H5OH): A benzene derivative with a hydroxyl group, utilized in plastic and pharmaceutical production.
Properties
Aromatic compounds are more stable due to the lack of reactivity of their electrons compared to their non-aromatic counterparts. Aromatic compounds typically undergo substitution reactions instead of addition reactions to maintain aromaticity, with electrophilic aromatic substitution being common. Many aromatic compounds, whether liquid or solid at room temperature, possess distinct odors and generally have higher boiling points compared to similar molecular weight aliphatic compounds.
Industrial Applications
Many aromatics, such as benzene, toluene, and xylenes, serve as solvents in chemical reactions and industrial processes. Aromatics are precursors for polymers such as polystyrene and polyesters. The aromatic structures are prevalent in many pharmaceuticals due to their stability and reactivity. Many dyes and pigments contain aromatic systems, providing vibrant and stable colors.
Process Description
Aromatics refer to cyclic hydrocarbon compounds that contain rings of six carbon atoms. Aromatic compounds, particularly benzene, toluene, and xylenes (collectively known as BTX), are primary feedstocks for producing numerous petrochemical intermediates, which in turn play roles in producing synthetic fibers, resins, explosives, pesticides, detergents, and many other materials.
The feedstock for catalytic cracking units comes from the atmospheric distillation of crude oil, which contains hydrocarbons from C6 to C10, including paraffinic, naphthenic, and aromatic compounds. Aromatic compound production occurs in petrochemical units that source their feedstock from crude oil refineries. Key processes in catalytic reforming include feed preparation, temperature control, reactions in the reformer, and product recovery. Two types of reformers available for this task are semi-regeneration and non-regeneration cyclic moving beds, and reactors can be selected among two types: radial flow and axial flow.
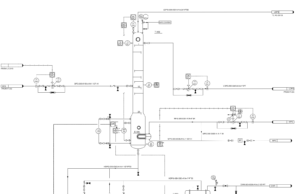
Catalytic Reforming
The reforming process is typically conducted in three or four adiabatic fixed-bed reactors. The catalysts used are mostly Pt/Al2O3, with some containing metals (Re, Ir, Sn) to enhance catalyst performance. Most catalytic reforming units operate as semi-regenerative systems, which means that after a shutdown period of 6-24 months, the coked catalysts are removed for recovery and the system is restarted after catalyst reactivation. Continuous catalyst recovery systems are also available, where a small amount of catalyst is continuously removed for recovery and then returned to the reactor, eliminating the need for shutdown and making it technically efficient.
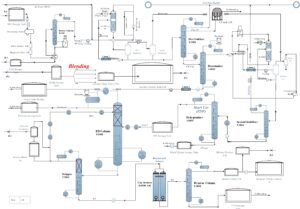
Separation of Aromatics
The raw pyrolysis gasoline (often referred to as Pyrolysis Gasoline) is directed to a solvent extraction unit following its output from the stripper. In this unit, aromatic compounds are separated from non-aromatic ones. The resulting aromatic-rich mixture is then introduced into a distillation column, where pure toluene and benzene are isolated. Following this, the output from the distillation column is processed in a secondary distillation unit to separate ethylbenzene and ortho-xylene from the remaining components. Finally, the mixture of ethylbenzene and ortho-xylene undergoes further separation in a splitter.
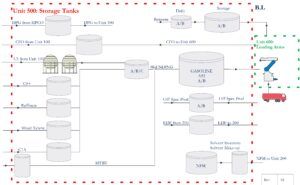
Unit 500 in an Industrial Petrochemical Complex
The diagram below provides an overview of Unit 500 in an industrial petrochemical complex. This unit acts as a central storage facility for various chemicals and plays a key role in the production of final products. Unit 500 consists of a series of tanks that accommodate various chemicals, designated either by abbreviations (such as DPG, CFO, C5) or full names (such as benzene, mixed xylenes).
The lines on the diagram indicate the movement pathways of chemicals between Unit 500 and other processing units (such as Units 100, 200, and 600). Some chemicals in this unit are mixed together to produce final products such as A92 gasoline. Unit 600 is designated as the loading unit, and final products are transferred to tankers or pipelines through loading arms. Unit 500 plays a crucial role in the production of final products. A comprehensive understanding of this unit’s operations will significantly contribute to improving efficiency, reducing costs, and enhancing safety.
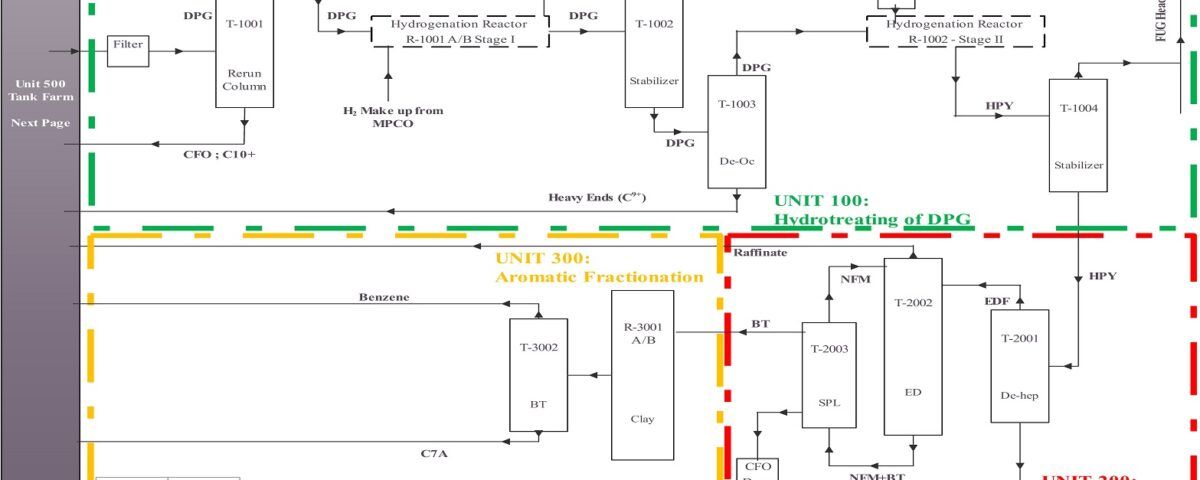
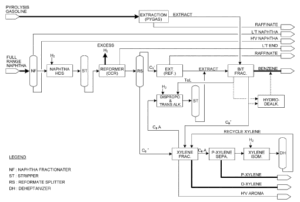
Technical Knowledge of Benzene Extraction and Gasoline Production From Pyrolysis Gasoline
Pyrolysis gasoline (Pygas) is a byproduct of the ethylene production process through steam cracking of hydrocarbons such as naphtha, diesel, or ethane. Pygas contains a mixture of hydrocarbons, predominantly aromatics, including benzene, toluene, and xylenes (BTX), which are valuable petrochemical materials.
Benzene Extraction From Pyrolysis Gasoline
1. Partial Distillation: Pygas is initially subjected to partial distillation to separate it into various fractions based on boiling points. Pygas is often stabilized to remove light compounds to prevent handling issues.
2. Hydrogenation: Pygas undergoes hydrotrituration to saturate olefins and diolefins, enhancing gasoline stability and reducing gum formation. This process is usually carried out using a catalyst under high temperature and pressure in the presence of hydrogen. Selective hydrogenation may be employed to target specific compounds while minimizing the hydrogenation of aromatic compounds.
3. Aromatic Extraction: This process separates benzene, toluene, and xylenes from non-aromatic components using a solvent that selectively dissolved aromatic compounds. Solvents such as sulfolane or N-methyl-2-pyrrolidone (NMP) are used for the selective extraction.
4. Distillation: After separation, the aromatic-rich stream undergoes partial distillation to purify benzene, toluene, and xylenes.

Conclusion
Aromatics, as organic compounds with benzene ring structures and unique physical and chemical properties, play a crucial role in various industries. Deep technical knowledge of these compounds is key to developing more efficient production processes, higher-quality products, and innovative applications. Aromatics are unique in structure and properties, significantly impacting our daily lives. Further research into these compounds can lead to a deeper understanding of our environment and contribute to technological advancements.
Providing Technical Knowledge of Aromatics to Petrosakht-Chehelsoton Company
APIPCO Company, leveraging deep technical knowledge, is capable of providing comprehensive solutions for benzene extraction from pyrolysis gasoline. The company has successfully executed multiple projects in this field, including:
1. Providing aromatic technical knowledge to Marun Petrochemical Company.
2. Delivering COMFAR and pre-feasibility studies.
3. Providing initial technical knowledge and feasibility study for the project implementation for Ilam Petrochemical and Petrol Company.